Abstract
Introduction: Angioimmunoblastic T-cell Lymphoma (AITL) is a type of peripheral T-cell lymphoma (PTCL) arising from CD4 positive follicular helper cells T-cells. Despite advances in treatment, data suggests an increased risk of development of second primary malignancies (SPMs) in patients with AITL. However, comprehensive population-based studies analyzing the incidence and spectrum of SPMs in AITL patients are lacking. In this study, we utilized the Surveillance, Epidemiology and End Results (SEER) database to evaluate the risk for SPMs in AITL cases.
Methods: We used the November 2021 submission of the SEER 17 registry, which covers ~26.5% of the US population based on the 2010 census as our study database. We used the SEER*Stat version 8.4.0 statistical software to analyze data. We identified cases diagnosed with AITL as their first primary malignancy between years 2000 and 2019 using International Classification of Diseases for Oncology edition 3 (ICD-O-3) code 9705/3 (angioimmunoblastic T-cell lymphoma) or Lymphoid neoplasm recode 2021 Revision 2(b)2.2.2. These cases were followed for several years, and the standardized incidence ratio (SIR) and absolute excess risk (AER) for development of SPMs in AITL cases were calculated.
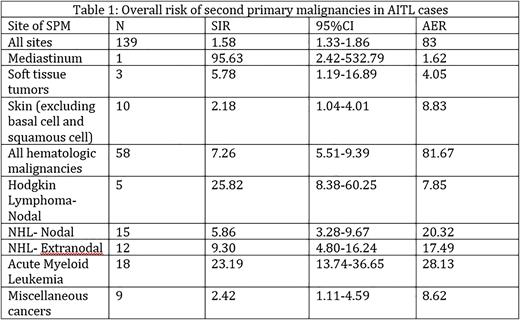
Results: A total of 1,632 cases were included in the SPM analysis, 899 (55%) males and 733 (45%) females. The study population comprised of 1303 (80%) whites, 130 (8%) blacks, 4 (0.3%) American Indians/Alaska Natives and 195 (12%) Asians/Pacific Islanders. Out of the entire cohort, 124 (8%) cases developed a total of 139 SPMs [SIR 1.58; 95% Confidence Interval (CI): 1.33-1.86] (Table 1). Median time to development of SPMs was 45 months. The overall risk of development of SPMs was especially higher 12-59 months (SIR 1.67; 95%CI 1.29-2.13) and 60-119 months (SIR 1.86; 95%CI 1.35-2.50) from diagnosis of AITL. 49% of SPMs that developed in the cohort were solid tumors but the overall risk of development of solid tumors was not significantly higher than that of [SIR 0.89; 95%CI 0.69-1.13] except for mediastinal tumors [SIR 95.63; 95%CI 2.42-532.79], soft tissue tumors [SIR 5.78; 95%CI 1.19-16.89] and skin cancers (excluding basal cell and squamous cell cancers) [SIR 2.18; 95%CI 1.04-4.01]. Interestingly, the risk of development of tumors of the nose and middle ear [SIR 45.92; 95%CI 1.16-255.88] and thyroid [SIR 9.33; 95%CI 1.13-33.69] was significantly higher 2-11 months from AITL diagnosis.
On the other hand, this population had a significantly higher overall risk of development of hematologic malignancies (SIR 7.26; 95%CI 5.51-9.39) as compared to the general population. This risk was especially elevated for Hodgkin Lymphoma (Nodal) [SIR 25.82; 95%CI 8.38-60.25], Non-Hodgkin Lymphoma (Nodal) [SIR 5.86; 95%CI 3.28-9.67], Non-Hodgkin Lymphoma (Extranodal) [SIR 9.30; 95%CI 4.80-16.24] and Acute Myeloid Leukemia [SIR 23.19; 95%CI 13.74-36.65].
Conclusion: To our knowledge, this is the first detailed population-based analysis of SPMs particularly in AITL patients. Our study shows that the incidence of SPMs is truly higher in AITL patients when compared with the general population. The risk is highest 12-119 months after diagnosis of AITL. We found a significantly increased risk for solid tumors of the mediastinum, soft tissue and skin cancers (excluding basal cell and squamous cell) and hematologic malignancies, specifically for Hodgkin (Nodal), Non-Hodgkin lymphoma (Nodal and Extranodal) and Acute Myeloid Leukemia compared with the general population. The results of our study are pertinent to guide survivorship and surveillance strategies among AITL patients. Whether the development of these SPMs is due to shared abnormalities of underlying molecular pathways or is treatment related is unclear. One limitation of our study is the lack of treatment data for these patients. Further studies are needed to understand the mechanisms leading to development of SPMs in AITL patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal